Part V in the series: The Future of Flexible Manufacturing
The key to manufacturing a consistent, quality product is the control strategy. Yet with several important things to consider when both defining a control strategy and scaling up an existing one, understanding the process and mitigating risk is crucial.
The basis of a good control strategy is a detailed understanding of the clinical performance of the product, its quality characteristics (CQAs and material attributes) and the related manufacturing process (CPPs). The benefits of implementing a control strategy are clear – it drives process performance qualification (PPQ) activities, executes the manufacturing process, and maintains the validated state throughout the product lifecycle.
Control strategy is also an important vehicle for robust manufacturing. It should be established during process development, and can include advanced PAT controls to ensure the product is manufactured to meet the expectations defined in the quality targeted product profile (QTPP).
Defining the state of control
To define control strategy, ICH Q8, Q9, and Q10 clearly identify that understanding, identifying and mitigating risk is key. For a truly flexible manufacturing operation, as depicted in Figure 1, there are points to consider in the design of a manufacturing facility, including both manufacturing and process control. These points include:
- How is the control strategy developed?
- How will the control strategy be updated/improved over the product manufacturing lifecycle?
- What is the optimal approach for change management in the control strategy evolution?
- What is the impact of scale in multi-product manufacturing on control strategy development?

Developing a control strategy
Developing a control strategy begins with a clear definition of the attributes defined in the QTPP. These attributes are measured and must comply with the justified acceptance criteria, linking back to the QTPP and the clinical performance of the product. You will then idenitgy CQAs for the product and the in-process materials, along with the CPPs for the baseline process.
For control strategy design, the following questions should be clearly answered:
- What attributes are important?
- What are the acceptance levels?
- Does the process control the CQAs within those levels?
- Are they stable?
- What needs to be tested?
- Do you have a robust process and testing strategy?
In most cases, the CQA acceptance criteria is formalized as product specifications. To facilitate control strategy development for multi-product facilities, it may be beneficial to begin with a generic set of CQAs and CPPs. However, you must establish a control strategy for each product; a control strategy is product-centric (Figure 2). This is because the master batch records and the control systems must reflect the product; it cannot be generic.

A science and risk-based approach identifies the material CQAs and CPPs of the manufacturing process using Design of Experiments (DoE) during development, with the major workload in clinical phases 2 and 3. The control strategy is the outcome of a risk assessment(s) where the controls implemented must be proportionate to the level of risk identified.
Updating and improving your control strategy
Highly integrated and flexible manufacturing facilities (see Figure 1) have important capabilities when rapidly commercializing new products. A manufacturing facility capable of making pilot through commercial scale material provides the following advantages:
- Minimal tech transfer – Because the same facility is used, manufacturing and process control strategies can be seamlessly scaled-up to supply early clinical material up to product launch quantities of product.
- Process validation – All three process validation steps (Design ICH Q8 design space, qualification and verification) can be coordinated and evolved as you scale up the process.
- Product demand – A flexible facility can rapidly scale-up and scale-out process trains to meet highly variable clinical and launch requirements.

Change management
One of the key focus areas around change management is the evaluation of risk and the impact of change. ICH Q10 provides the basis for change management, while applying Q8 and Q9 principles provides opportunities to optimize the control strategy (Figure 4).
While the QMS defines procedures for change evaluation by cross-functional teams or disciplines, be aware that you must support the technical justification for the change by using the proper risk tool that identifies any additional activities. With multi-product/multi-phase manufacturing, this includes:
- Quality (individual product)
- Safety, identity, strength, purity
- Efficacy
- Regulatory notification

In addition to adherence to ICH Q10, a change review board can establish KPIs and acceptance criteria in a systematic manner. This manages the impact to the overall commercial enterprise.
Integrated engineering approach
Understanding that the control strategy is an integral part of the product’s development life cycle opens up considerations about the required engineering activities to rapidly commercialize a product. Therefore, we must examine the practical application of the control strategy from the perspective of process and production system engineering. The value of the control strategy lies in it being implemented as the foundation of integrated engineering and design activities towards commercialisation. To support this process, there must be a common understanding from all parties involved. This includes drug development, facility engineering, process engineering and automation engineering. The resulting multi-disciplinary product specifications must be developed as a set of integral activities.
The control strategy also needs to include requirements for the product, such as how it should be produced and with what equipment, and how the equipment and process should be automated and controlled. This can only be achieved using an integrated engineering approach - where development activities that define the product’s quality attributes, production processes, equipment and their control and automation, run simultaneously. One sure way to achieve the intended design goals and effectively use the control strategy is to adopt a concurrent engineering approach - not only because it fulfils the objectives of the approach but also because it has been proven to work in other industries.
The impact of scale
It is also important to consider the risk associated with a scale-up of control strategy development to maximize the probability of effectiveness. The design and need for scale-up studies can depend on the development approach used and the knowledge available. Applying a risk-based approach can help assess the suitability of a control strategy across different scales. QRM tools should be used to guide these activities.
Some of the key considerations in control strategy development for scale-up considerations include:
1. Complexity of product and process
- Any differences in manufacturing equipment, facilities and/or sites
2. Raw materials:
- Any differences in raw material quality due to source or batch-to-batch variability
- Impact of such differences on process controls and quality attributes
3. Process parameters:
- Confirmation or optimization
- Confirmation of the design space(s), if used
4. In-process controls:
- Point of control
- Optimization of control methods
- Optimization and/or updating of models, if used
5. Product specification:
- Verification of the link to QTPP
- Confirmation of specifications (i.e., methods and acceptance criteria)
- Confirmation of real-time release testing (RTRT), if used
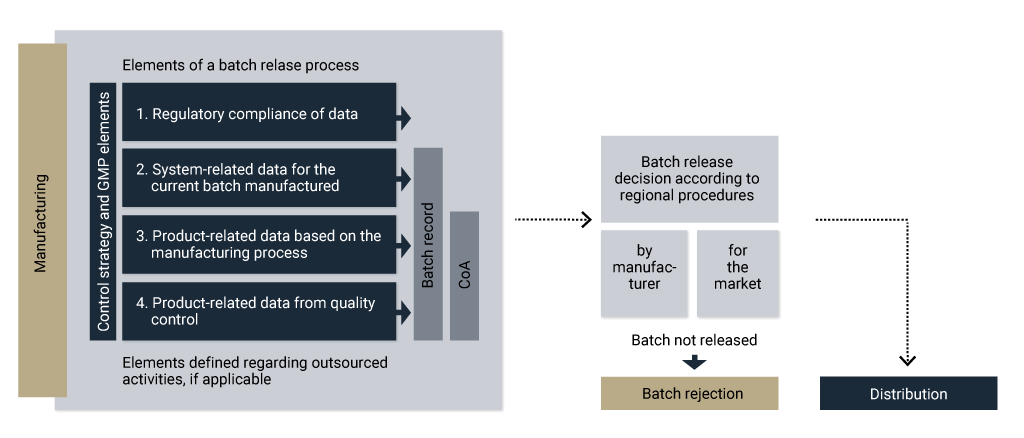
Batch release for drug substance
Different development approaches lead to different control strategies. Regardless of the control strategy, the batch release process should be followed. For a batch release decision, you should consider several manufacturing elements, illustrated in Figure 4.
To sum up, developing a strong and clearly defined control strategy that can be improved over the lifecycle of products is extremely beneficial - offering up new realms of manufacturing flexibility to your facility.




